New type of CT scanner approved by FDA
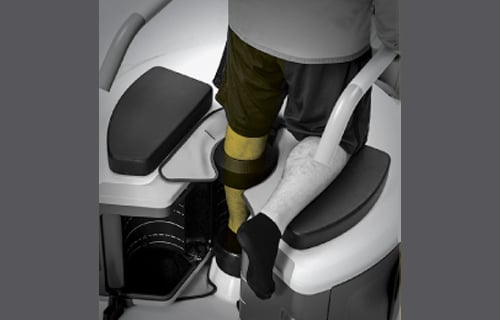
Jeffrey Siewerdsen, professor of biomedical engineering, and a team of researchers have designed a new type of CT scanner that has recently been approved for commercial use by the U.S. Food and Drug Administration.
Rather than lying flat, patients are able to stand as the scanner examines the joints in their feet, ankles, or knees. Since the joints are bearing weight in this position, health care workers are able to identify issues that may otherwise go unnoticed.
Developed in collaboration with Carestream Health in Rochester, New York, the new scanner delivers higher-quality images at lower levels of radiation, allowing clinics to install them without shielded rooms. In addition to showing fine bone details, the new scanner is able to display cartilage, tendons, and ligaments as well.
The scanner was invented through an academic-industry partnership and translated into clinical trials at the Johns Hopkins Hospital. Johns Hopkins Technology Ventures handled the exclusive licensing deal.
Click here for the story in JHU Engineering Magazine.
