New epilepsy tool could cut misdiagnoses by nearly 70% using routine EEGs
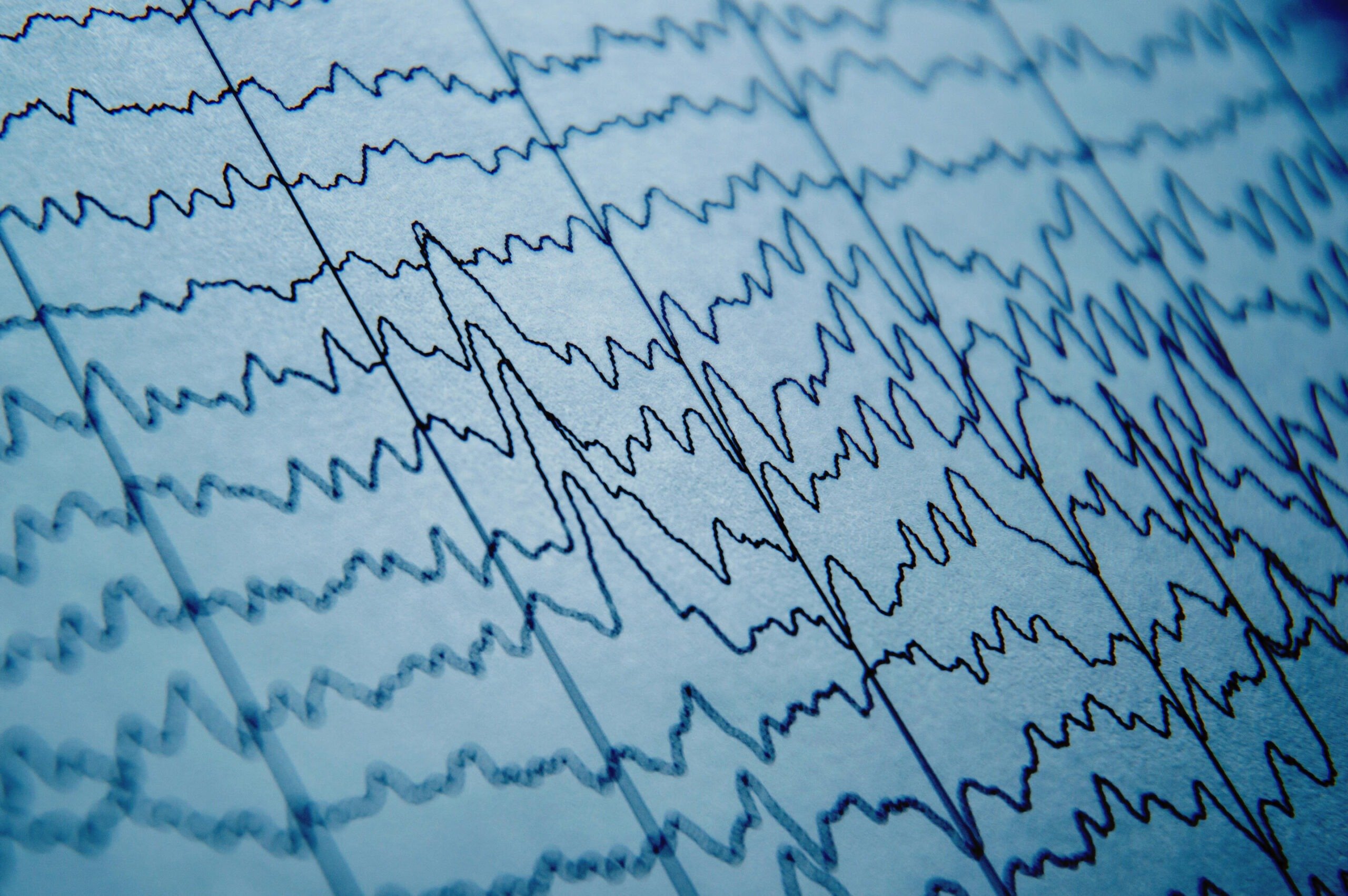
Doctors could soon reduce epilepsy misdiagnoses by up to 70% using a new tool that turns routine electroencephalogram, or EEG, tests that appear normal into highly accurate epilepsy predictors, a Johns Hopkins University study has found.
By uncovering hidden epilepsy signatures in seemingly normal EEGs, the tool could significantly reduce false positives—seen in around 30% of cases globally—and spare patients from medication side effects, driving restrictions, and other quality-of-life challenges linked to misdiagnoses.
“Even when EEGs appear completely normal, our tool provides insights that make them actionable,” said Sridevi V. Sarma, a Johns Hopkins biomedical engineering professor who led the work. “We can get to the right diagnosis three times faster because patients often need multiple EEGs before abnormalities are detected, even if they have epilepsy. Accurate early diagnosis means a quicker path to effective treatment.”
A report of the study is newly published in Annals of Neurology.
Epilepsy causes recurrent, unprovoked seizures triggered by bursts of abnormal electrical activity in the brain. Standard care involves scalp EEG recordings during initial evaluations. These tests track brainwave patterns using small electrodes placed on the scalp.
Clinicians partly rely on EEGs to diagnose epilepsy and decide whether patients need anti-seizure medications. However, EEGs can be challenging to interpret because they capture noisy signals and because seizures rarely occur during the typical 20 to 40 minutes of an EEG recording. These characteristics makes diagnosing epilepsy subjective and prone to error, even for specialists, Sarma explained.
To improve reliability, Sarma’s team studied what happens in the brains of patients when they are not experiencing seizures. Their tool, called EpiScalp, uses algorithms trained on dynamic network models to map brainwave patterns and identify hidden signs of epilepsy from a single routine EEG.
“If you have epilepsy, why don’t you have seizures all the time? We hypothesized that some brain regions act as natural inhibitors, suppressing seizures. It’s like the brain’s immune response to the disease,” Sarma said.
The new study analyzed 198 epilepsy patients from five major medical centers: Johns Hopkins Hospital, Johns Hopkins Bayview Medical Center, University of Pittsburgh Medical Center, University of Maryland Medical Center, and Thomas Jefferson University Hospital. Out of these 198 patients in the study, 91 patients had epilepsy while the rest had non-epileptic conditions mimicking epilepsy.
When Sarma’s team reanalyzed the initial EEGs using EpiScalp, the tool ruled out 96% of those false positives, cutting potential misdiagnoses among these cases from 54% to 17%.
“This is where our tool makes a difference because it can help us uncover markers of epilepsy in EEGs that appear uninformative, reducing the risk of patients being misdiagnosed and treated for a condition they don’t have,” said Khalil Husari, co-senior author and assistant professor of neurology at Johns Hopkins. “These patients experienced side effects of the anti-seizure medication without any benefit because they didn’t have epilepsy. Without the correct diagnosis, we can’t find out what’s actually causing their symptoms.”
In certain cases, misdiagnosis happens due to misinterpretation of EEGs, Husari explained, as doctors may overdiagnose epilepsy to prevent the dangers of a second seizure. But in some cases, patients experience nonepileptic seizures, which mimic epilepsy. These conditions can often be treated with therapies that do not involve epilepsy medication.
In earlier work, the team studied epileptic brain networks using intracranial EEGs to demonstrate that the seizure onset zone is being inhibited by neighboring regions in the brain when patients are not seizing. EpiScalp builds on this research, identifying these patterns from routine scalp EEGs.
Traditional approaches to improve EEG interpretation often focus on individual signals or electrodes. Instead, EpiScalp analyzes how different regions of the brain interact and influence one another through a complex network of neural pathways, said Patrick Myers, first author and doctoral student in biomedical engineering at Johns Hopkins.
“If you just look at how nodes are interacting with each other within the brain network, you can find this pattern of independent nodes trying to cause a lot of activity and the suppression from nodes in a second region, and they’re not interacting with the rest of the brain,” Myers said. “We check whether we can see this pattern anywhere. Do we see a region in your EEG that has been decoupled from the rest of the brain’s network? A healthy person shouldn’t have that.”
The team is now conducting a larger prospective study to further validate its findings across three epilepsy centers and filed a patent for the EpiScalp technology in 2023.
Other authors are Kristin Gunnarsdottir, Adam Li, Alana Tillery, Babitha Haridas, and Joon-yi Kang of Johns Hopkins; Vlad Razskazovskiy, Jorge Gonzalez-Martinez, and Anto Bagíc of University of Pittsburgh Medical Center; Dale Wyeth, Edmund Wyeth, and Michael Sperling of Thomas Jefferson University Hospital; Kareem Zaghloul and Sara Inati of the National Institute of Neurological Disorders and Stroke, National Institutes of Health; Jennifer Hopp of University of Maryland Medical Center; and Niravkumar Barot of Beth Israel Deaconess Medical Center.
The research was supported by the Louis B. Thalheimer Fund for Translational Research at Johns Hopkins Technology Ventures, as well as the National Institute of Neurological Disorders and Stroke Grant Number: R35NS132228.
