Meet RoboDrop, the superbug slayer
During January’s World Economic Forum in Davos, Switzerland, experts cautioned that by 2050, as many as 10 million people a year could die from drug-resistant bacteria, viruses, and other bugs. Antibiotic resistance already kills more than a million people a year, and public health officials warn the problem will get worse.
One strategy to combat resistance is the development of new combinations of existing antibiotics that work together to pack a stronger punch. However, testing these mixtures is challenging because of the large number of possible combinations, the potential for dangerous interactions, and the cost.
Johns Hopkins researchers have developed a possible solution called RoboDrop: a robotic platform capable of rapidly screening multiple combinations of antibiotics simultaneously to find the most potent mixtures. Their paper, “Automated and miniaturized screening of antibiotic combinations via robotic-printed combinatorial droplet platforms” appears in Acta Pharmaceutica Sinica B.
“RoboDrop has tremendous promise as an automated platform for efficiently evaluating antibiotic combinations against drug-resistant bacteria. In our current phase, we focused on testing the model E. coli bacteria strain, demonstrating success in screening thousands of droplets over a few hours and identifying several effective synergistic combinations,” said Jeff Wang, senior author of the study and a professor of mechanical engineering at Johns Hopkins Whiting School of Engineering.
For example, during testing, RoboDrop discovered a three-drug pairing that fully eliminated the E. coli strain at concentrations against which individual antibiotics had no effect. The team also cultured bacteria that survived the drug cocktails. This allowed them to track the efficacy of the combinations at different time points and gain insights into the underlying process.
“This technology not only quickly streamlines the identification of synergistic combinations, but it also provides a tool for understanding and navigating the complexities of higher-order antibiotic interactions,” said Fangchi Shao, lead author and a doctoral candidate in biomedical engineering at School of Medicine. “The possible beneficiaries of this new technology are vast, including healthcare professionals, researchers, and – ultimately – patients.”
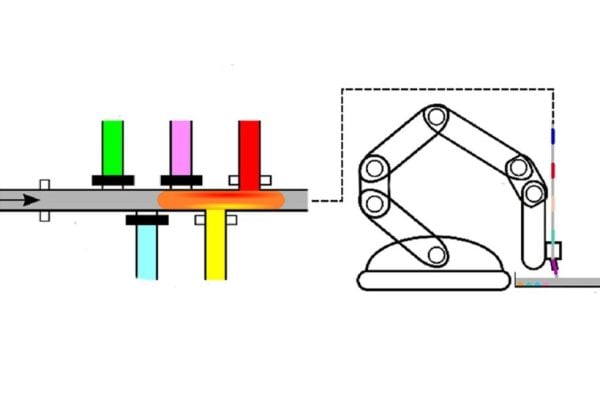
The compact device comprises three parts: a microfluidic chip that produces tiny droplets of antibiotic mixtures, a tube that ferries the droplets to the robotic arm, and an arm that precisely prints the complex arrangements of droplets onto Petri dishes. Because it uses droplets 20,000 times smaller than a teardrop, the platform significantly reduces the amount of sample liquids needed. The team also outfitted RoboDrop with customized software that allows it to run experiments and analyze data on its own.
“By speeding up the process of identifying effective antibiotic combinations, our work could greatly improve patient outcomes, enhance treatment efficacy, and contribute to broader efforts to combat multidrug-resistant bacteria,” Shao said. “Additionally, we envision it driving the development of new treatment strategies and the optimization of existing antibiotics, turning the tide against superbugs and saving countless lives threatened by antimicrobial resistance worldwide.”
The upcoming phase of the team’s research will specifically examine combinations effective against resistant bacteria, assessing the adaptability of the system to different bacterial strains encountered in real-world scenarios. This strategic expansion aims to enhance the versatility and applicability of our platform, making it a valuable tool across various medical settings.
Co-authors include Kuangwen Hsieh, Hui Li, and Sixuan Li in the Department of Mechanical Engineering and Pengfei Zhang in the Department of Biomedical Engineering. The project was supported by the National Institutes of Health.
