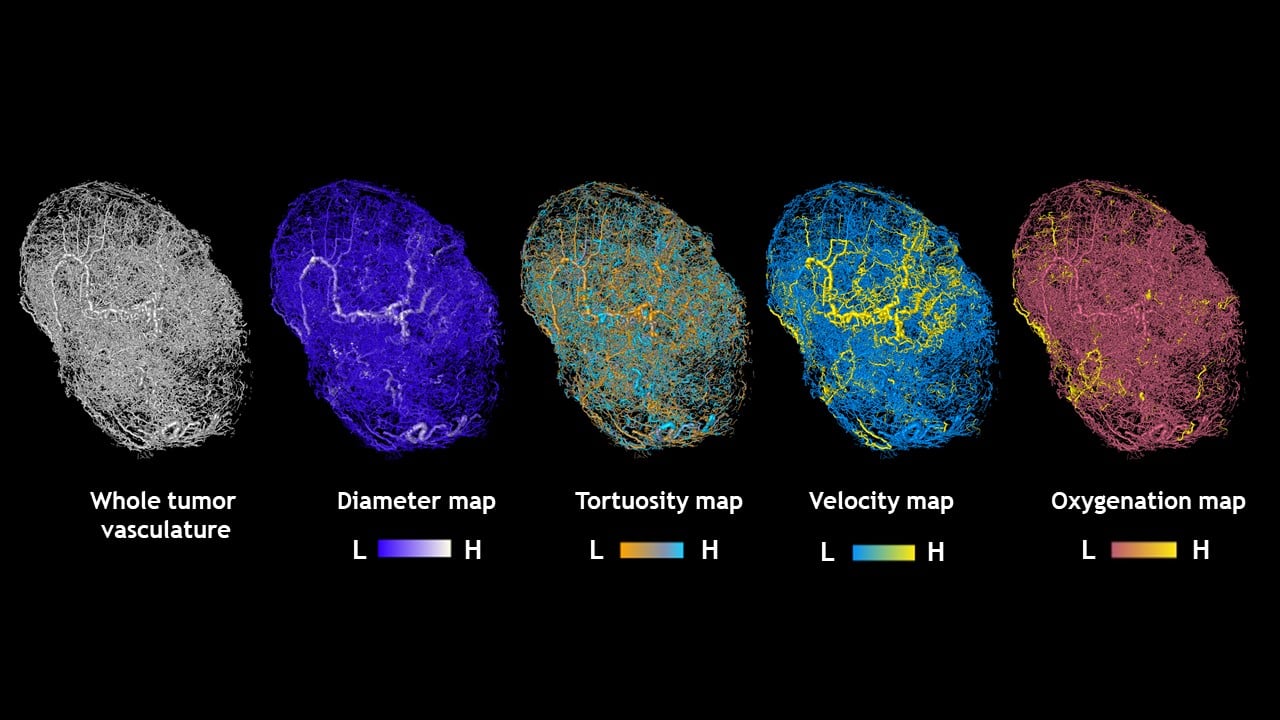
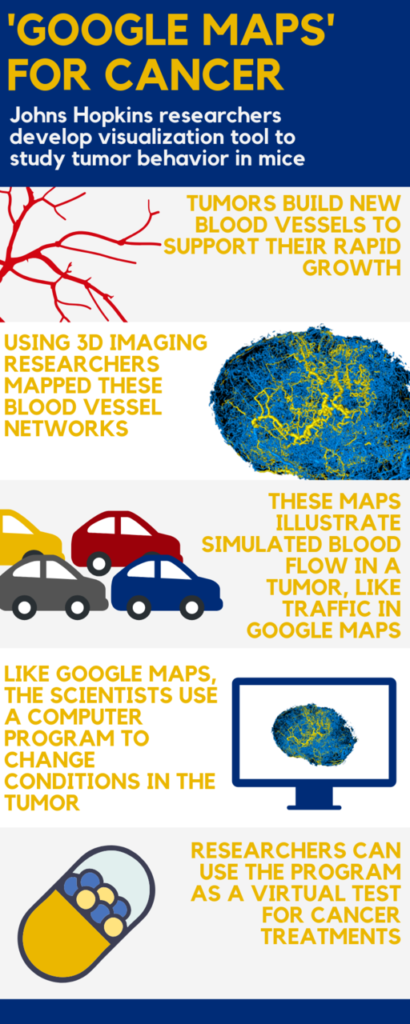
Johns Hopkins Medicine researchers say they have developed something akin to a “Google Maps” approach for more accurately computing and visualizing the structural and functional blood vessel changes needed for tumor growth. By pairing high-quality 3D imaging data of tumor specimens from animal models with sophisticated mathematical formulas, the researchers say they now have a model that accurately represents blood traffic inside tumors, including the complex blood flow, oxygenation and structural changes that occur.
This work was published March 27 in Nature Scientific Reports.
Arvind Pathak, Ph.D., the study’s leader, says the project was developed out of the need to address the complexities of cancer biology in growing and developing tumors. He saw the need for better predictive models, as many existing models employed rudimentary approximations of the complex geometry of a tumor’s blood vessels.
“Poor representations of the tumor ‘microenvironment’ make models and the research based on them less accurate. So I thought, how I can use my imaging expertise to improve the information available to modelers?” says Pathak, associate professor of radiology and biomedical engineering at the Johns Hopkins University School of Medicine and a member of the Johns Hopkins Kimmel Cancer Center.
To create a model that more precisely reflected the structure and behavior of tumors, his research team implanted mice with human breast tumor cells and imaged the resulting tumors using 3D magnetic resonance microscopy and micro-CT imaging. These high-resolution 3D images provided detailed information about a tumor’s volume and the structure of its blood vessel networks.
With the underlying blood vessel structure mapped, Pathak and his research team scoured the scientific literature to find information on how these structures would likely behave in a living system. Specifically, they searched for studies that included measurements of blood pressure, blood flow and volume in vessels similar to those seen in their tumors.
Using this information, the group collaborated with Aleksander Popel’s Systems Biology Laboratory in the Department of Biomedical Engineering at the Johns Hopkins University School of Medicine to develop a set of mathematical formulas designed to represent these aspects of tumors. They did this by combining data from previously published studies with the information they collected.