Recent Developments:
Other News: pending.
Grant Accelerator Award
Doloff Lab receives $15,000 Core facilities Grant accelerator award!
Three from Hopkins BME earn Johns Hopkins Catalyst Award

Thirty-five promising early-career faculty members from across the nine academic divisions of Johns Hopkins University have been selected to receive Johns Hopkins Catalyst Awards for 2024, a prestigious recognition reflecting their accomplishments to date, creativity and originality, and academic impact. Among the award winners this year are Joshua Doloff, Jude Phillip, and Jeremias Sulam, all three are assistant professors of biomedical engineering.
Doloff’s research focuses on exploring the intersection between living systems and biologic or synthetic therapeutics to better understand what happens when deliverables are introduced into the body and how the host immune system responds. …. FULL STORY HERE
‘Artificial Lymph Node’ Used to Treat Cancer in Mice

Johns Hopkins Medicine scientists say they have developed an artificial lymph node with the potential to treat cancer, according to a new study in mice and human cells. The newly developed lymph node — a sac filled with immune system components — is implanted under the skin, and is designed to act like a learning hub and stimulator to teach immune system T-cells to recognize and kill cancer cells.
Details of the experiments are published recently online and in the June 6 issue of Advanced Materials.
Joshua Doloff receives grant from Ovarian Cancer Research Alliance

Joshua Doloff, an assistant professor in the Department of Biomedical Engineering, haswon a three-year, $450,000 grant from Ovarian Cancer Research Alliance to design new drug delivery systems for more precise treatment of ovarian cancer.
Doloff was chosen as one of six junior faculty nationwide to receive the OCRA’s Early Career Investigator Grant. Doloff’s research focuses on exploring the intersection between therapeutics, whether biologic or synthetic in origin, and living systems to better understand what happens when deliverables are introduced into the body and, of major importance, how the host immune system behaves (e.g., helps or interferes with) upon perceiving them. For this project, Doloff and his team are working to develop innovative crystalline drug reservoirs and biomaterial deposition systems to accomplishprecision delivery in treating ovarian cancer. FULL STORY HERE
New immune model sheds light on implant rejection
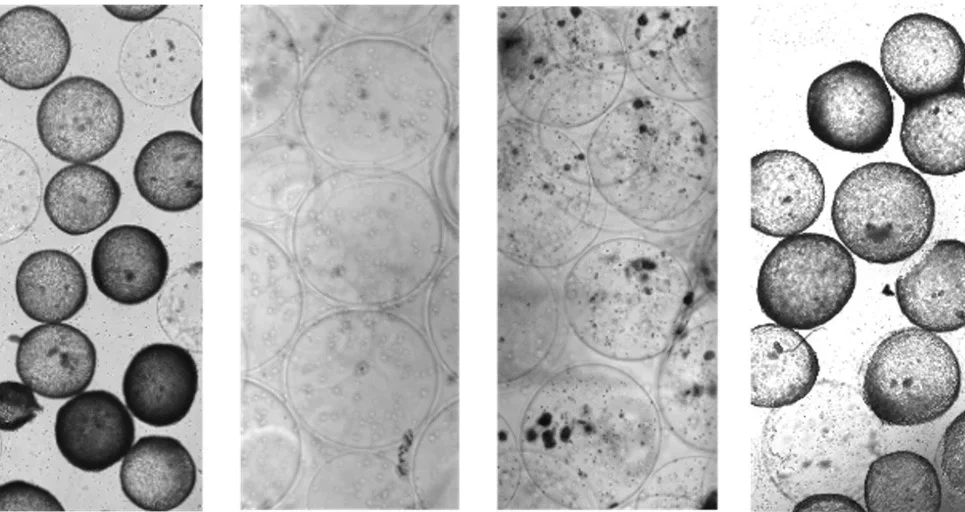
Implantable medical devices–think artificial joints, cochlear implants, and insulin pumps–make some of our most challenging health issues more manageable. Even so, human bodies frequently reject implanted biomaterials, and currently, there are few ways to predict or prevent it from happening.
A team of researchers led by Joshua Doloff, an assistant professor of biomedical engineering, has discovered an advanced “humanized” mouse model to better predict how the human immune system will react to medical implants. Their results appear in Science Advances. …. FULL STORY HERE
Smoothing the Way to Better Breast Implants

Silicone breast implants with a smoother surface design have less risk of producing inflammation and other immune system reactions than those with more roughly textured coatings, according to new research by Joshua Doloff, assistant professor of biomedical engineering, who collaborated with researchers at Johns Hopkins Medicine, MIT, and Rice University on a series of experiments published last June in Nature Biomedical Engineering. …. FULL STORY HERE
Recent lab collaboration and funding from the JDRF Highlighted! (LINK)
Congrats to Dr. Doloff for recently being awarded a Young Investigator Award from the Controlled Release Society’s Immuno Delivery Group!




Check out the Future (and quotes from numerous professors including Dr. Doloff) in Hopkins BME News’ “Tissue Engineering: The Future Is Here“
Congrats to Dr. Doloff for being recognized as a 2021 BMES CMBE Young Innovator!


Two Press Releases: MIT (LINK) & Hopkins (LINK), on our recent Nature BME Breast Implant study focusing on a new surface topography for ameliorating fibrosis/foreign body response.
Congrats to PhD Student Stuart Bauer for our new News & Views article out in Nature Biomedical Engineering: CHECK IT OUT!
Congrats to PhD Student Jessica Stelzel for our new News & Views article out in Nature Materials: CHECK IT OUT!
We are honored to be a part of a large Johns Hopkins School of Medicine team that recently received a DARPA award for investigating ultrasound/Doppler implant systems for spinal cord injury and repair! (Continue reading just below)
Cross-disciplinary team will design, develop devices to better treat spinal cord injuries
Funded through the Defense Advanced Research Projects Agency, the project will bring together experts from across Johns Hopkins to create solutions that work on the battlefield and on the frontlines of health care.
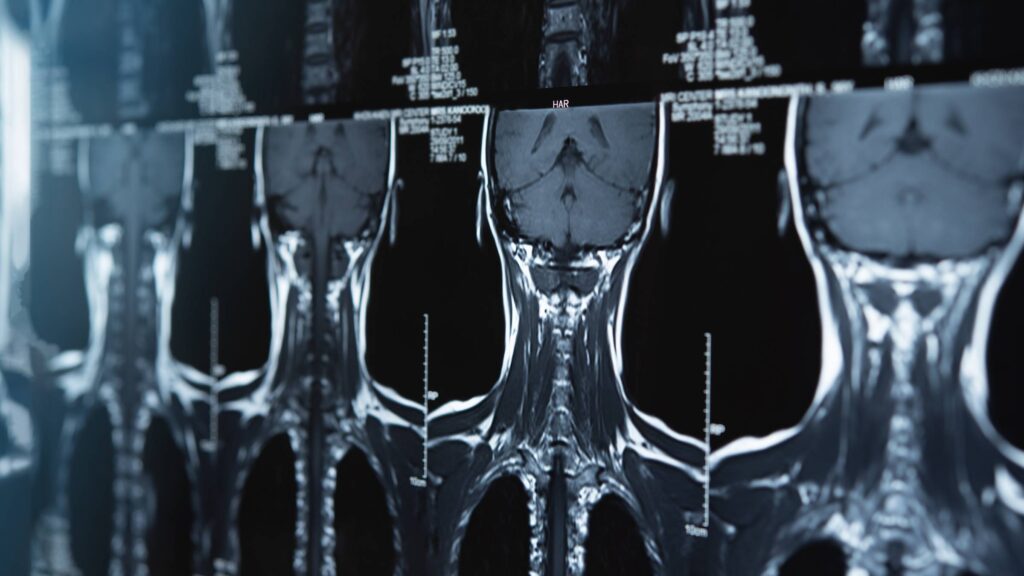
A team of Johns Hopkins biomedical engineers and neurosurgeons has received $13.48 million from the Defense Advanced Research Projects Agency to develop implantable ultrasound and other devices that could revolutionize care for people suffering from spinal cord injuries. The results could benefit thousands of U.S. service members and civilians who sustain spinal cord injuries every year…. FULL STORY HERE
Drug crystals to prevent medical device fibrosis
Crystallized drug prevents immune system rejection of transplanted pancreatic islet cells.
Implanted medical devices can save lives, but can also put patients at the risk of fibrosis, a condition in which the immune system attacks the device and produces scar tissue around it, interfering with the device’s functionality. Working with researchers at Massachusetts Institute of Technology, Joshua Doloff, an assistant professor of biomedical engineering at Johns Hopkins University and former MIT postdoc, has devised a new way to prevent fibrosis: loading implantable devices with a crystallized immunosuppressant drug…. FULL STORY HERE
Faculty Highlight: Meet Joshua Doloff, Assistant Professor of BME

Joshua Doloff joined the Johns Hopkins Department of Biomedical Engineering as an assistant professor in November 2018. In this interview, Doloff, who has an interest in technology and biology, describes his eagerness to build research collaborations and provide mentorship to students. He also discusses his “eureka moments,” the research he plans to conduct at Hopkins, and the future of engineering…. FULL STORY HERE
Oxygen-tracking method could improve diabetes treatment
Measurements could help scientists develop better designs for a bioartificial pancreas.

Transplanting pancreatic islet cells into patients with diabetes is a promising alternative to the daily insulin injections that many of these patients now require. These cells could act as a bioartificial pancreas, monitoring blood glucose levels and secreting insulin when needed…. FULL STORY HERE
Study points a way to better implants
Selectively blocking immune cells can prevent formation of scar tissue around medical devices.
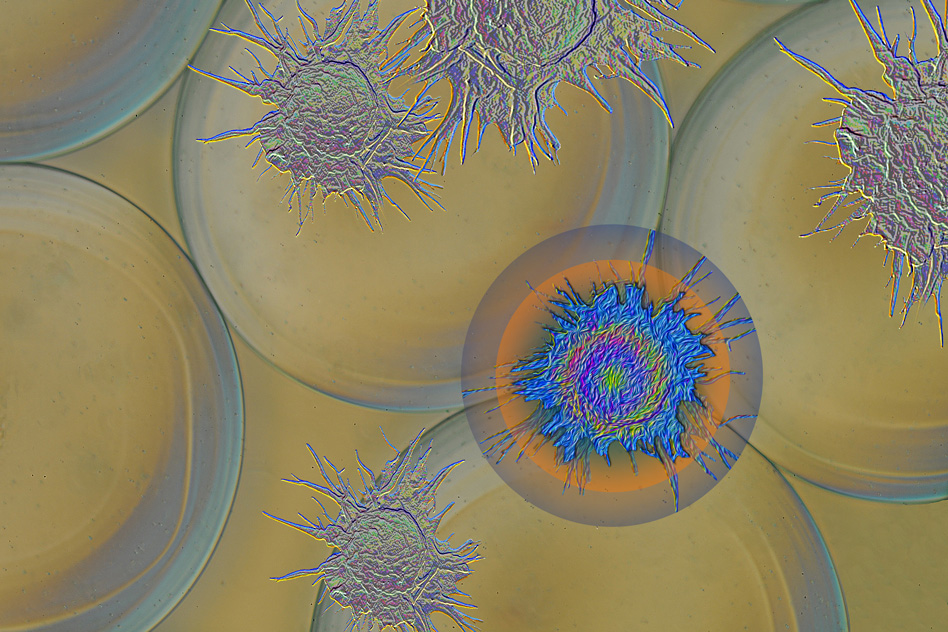
Medical devices implanted in the body for drug delivery, sensing, or tissue regeneration usually come under fire from the host’s immune system. Defense cells work to isolate material they consider foreign to the body, building up a wall of dense scar tissue around the devices, which eventually become unable to perform their functions… FULL STORY HERE
Scientists have figured out how to stop our bodies from fighting electronic implants
Scar tissue may no longer be an obstacle on our road to becoming cyborgs

We may dream of one day becoming cyborgs, with RFID chips implanted in our hands or bionic eyes, but the human body won’t necessarily cooperate. Implantable devices like pacemakers have been used regularly for decades, but they often encounter a build-up of scar tissue, or fibrotic tissue around the implants that can hinder functionality. Now, biologists have figured out a way to fight that process without seriously compromising the entire immune system…. FULL STORY HERE
Designing better medical implants
Optimal size and shape allow implantable devices to last longer in the body.
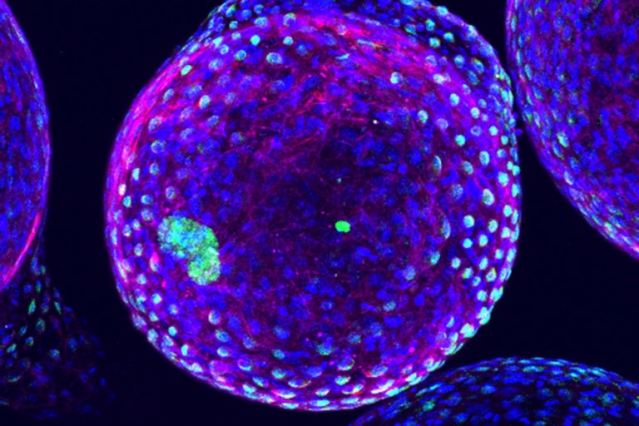
Biomedical devices that can be implanted in the body for drug delivery, tissue engineering, or sensing can help improve treatment for many diseases. However, such devices are often susceptible to attack by the immune system, which can render them useless.
A team of MIT researchers has come up with a way to reduce that immune-system rejection. In a study in Nature Materials, they found that the geometry of implantable devices has a significant impact on how well the body will tolerate them… FULL STORY HERE

