Johns Hopkins scientists who arranged for 48 human bioengineered heart tissue samples to spend 30 days at the International Space Station report evidence that the low gravity conditions in space weakened the tissues and disrupted their normal rhythmic beats when compared to earth-bound samples from the same source.
The scientists said the heart tissues “really don’t fare well in space,” and over time, the tissues aboard the space station beat about half as strong as tissues from the same source kept on Earth.
The findings, they say, expand scientists’ knowledge of low gravity’s potential effects on astronauts’ survival and health during long space missions, and they may serve as models for studying heart muscle aging and therapeutics on Earth.
A report of the scientists’ analysis of the tissues will be published during the week of Sept. 23 in the Proceedings of the National Academy of Sciences.
Previous studies showed that some astronauts return to Earth from outer space with age-related conditions, including reduced heart muscle function and arrhythmias (irregular heartbeats), and that some, but not all, effects dissipate over time after their return.
But scientists have sought ways to study such effects at a cellular and molecular level in a bid to find ways to keep astronauts safe during long spaceflights, says Deok-Ho Kim, a professor of biomedical engineering and medicine at Johns Hopkins University. Kim led the project to send heart tissue to the space station.
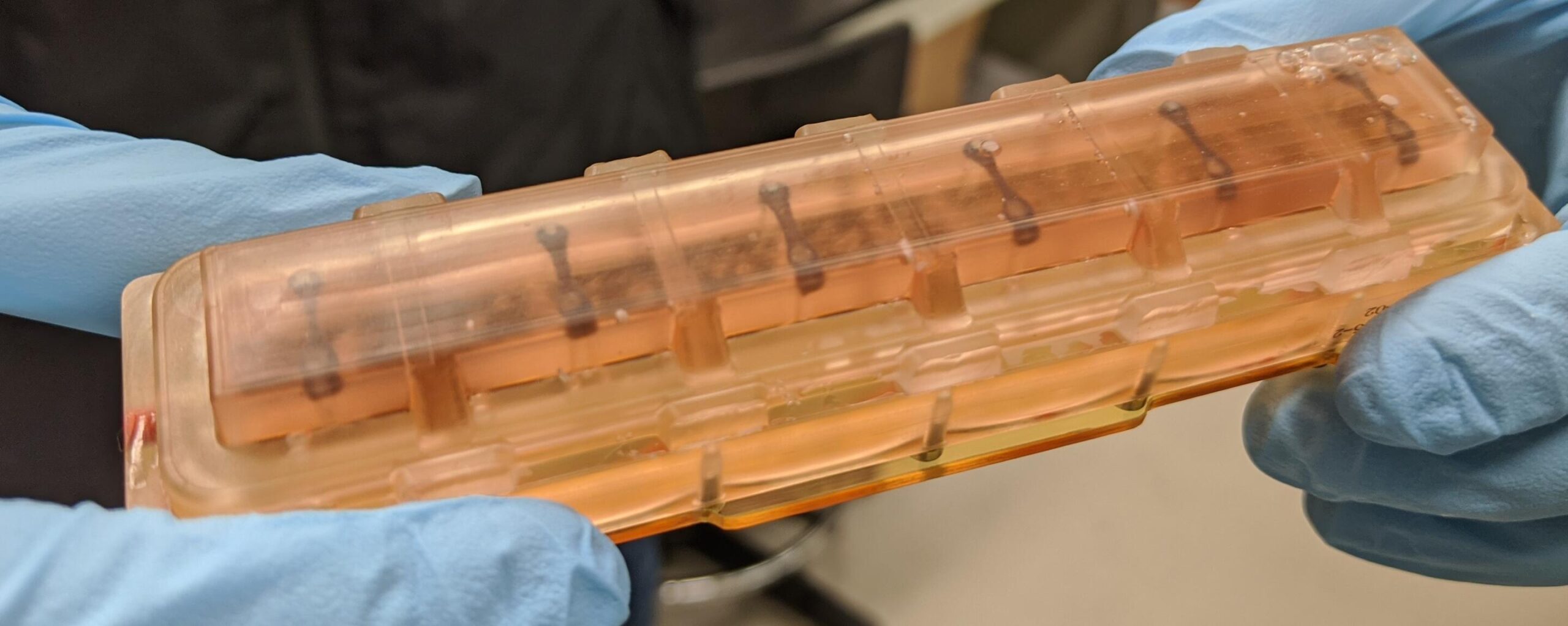
To create the cardiac payload, scientist Jonathan Tsui, coaxed human induced pluripotent stem cells (iPSCs) to develop into heart muscle cells (cardiomyocytes). Tsui, who was a PhD student in Kim’s lab at the University of Washington, accompanied Kim as a postdoctoral fellow when Kim moved to Johns Hopkins University in 2019. They continued the space biology research at Johns Hopkins.
Tsui then placed the tissues in a bioengineered, miniaturized tissue chip that strings the tissues between two posts to collect data about how the tissues beat (contract). The cells’ 3D housing was designed to mimic the environment of an adult human heart in a chamber half the size of a cell phone.
To get the tissues aboard the SpaceX CRS-20 mission, which launched in March 2020 bound for the space station, Tsui says he had to hand carry the tissue chambers on a plane to Florida, and continue caring for the tissues for a month at the Kennedy Space Center. Tsui is now a scientist at Tenaya Therapeutics, a company focused on heart disease prevention and treatment.
Once the tissues were on the space station, the scientists received real-time data for 10 seconds every 30 minutes about the cells’ strength of contraction, known as twitch forces, and on any irregular beating patterns. Astronaut Jessica Meir changed the liquid nutrients surrounding the tissues once each week and preserved tissues at specific intervals for later gene readout and imaging analyses.
The research team kept a set of cardiac tissues developed the same way on Earth, housed in the same type of chamber, for comparison with the tissues in space.